I've been on Planet Crooter for just over a week now (one Crooter week that is, or 8.4 Eazrah days) and have had a chance to find out more about iron fertilization.
The more I learn, the less impressive it seems.
My last entry outlined the basic concept and pointed to a number of studies showing some evidence for carbon sequestration as a result of iron fertilization of the oceans.
It turns out that legal experts have also claimed that HSRC (an organisation responsible for dumping 100 tonnes of iron dust in the Pacific Ocean) violated two UN conventions – the convention on biological diversity (CBD) and the London convention on dumping wastes at sea - thus breaking international law.
However, Freestone and Rayfuse (2008) note that iron fertilization violates laws on dumping waste in the ocean "unless and until" rigorous scientific research can demonstrate that the benefits of doing so would "outweigh the risks to the marine environment". So, if there are long-term benefits then perhaps (and I stress the word perhaps here), in very carefully controlled conditions, iron fertilization could be deemed acceptable in the eyes of international law.
Predictions of long-term iron fertilization
It seems that on a large scale, to see any significant effect over a prolonged period iron fertilization would have to be performed continuously (Aumont and Bopp, 2006).
Cao and Caldeira (2010) have run predictions to see what we could hope to gain from continuous iron fertilization. They reckon that by 2100, "globally sustained ocean iron fertilization" could have reduced atmospheric carbon dioxide from 965 ppm (the Intergovernmental Panel on Climate Change's (IPCC) A2 emission scenario for 2100) to 833 ppm. That's a 14% reduction. Good, some might say, but not good enough. Especially considering this estimation is highly optimistic. (Not least because Cao and Caldeira's predictions assumed sustained global iron fertilization since 2008!) This prediction is fairly consistent with Aumont and Bopp's (2006) claim that long term carbon sequestration from iron fertilization is negligible.
But, even if iron fertilization doesn't seem jaw-droppingly impressive as a means of carbon sequestration, I keep wondering: is something still better than nothing? Could it still be part of a wider plan to reduce atmospheric CO2?
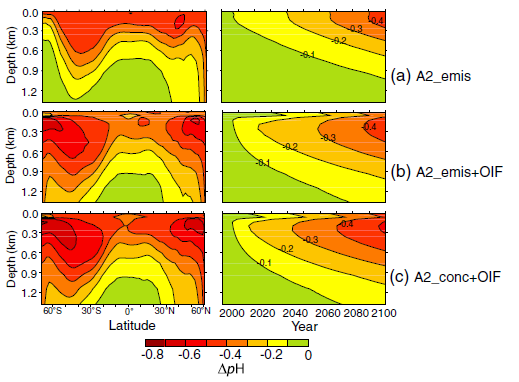
The answer to this, I’m afraid, is no. Even such reductions in atmospheric CO2 as were predicted by Cao and Caldeira (2010) or Aumont and Bopp (2006) would come at a high price elsewhere, deeper down. Carbon could be sequestered and stored in the deep ocean but, Cao and Caldeira warn, this would lead to a rise in deep-sea CO2 concentrations, and risk serious deep-sea ocean acidification (see Figure 1).
Deep sea ocean acidification is dangerous, for one thing, but it could also be totally counterproductive to the efforts of iron fertilization that caused it in the first place. Oceans are in constant motion: waves, tides, currents, mixing, and upwelling (just to name a few of the things they do). Upwelling is basically the process of deep water moving upwards, to replace surface water that's blown away by winds. If deep water is acidic (as predicted by Cao and Caldeira (2010)), then upwelling will eventually mean this deep acidic water (acidic with dissolved CO2) reaches the surface again, and it will be less able to take in much CO2 from the atmosphere.
Quite a hazardous outcome, and certainly not one that could be deemed beneficial enough to see a lifting of the international ban on dumping waste in oceans!
And as if this isn’t convincing evidence against iron fertilization, Aumont and Bopp (2006) note that when iron fertilization is stopped sequestered carbon is re-exposed to the atmosphere quite rapidly, again undoing all the hard work.
This, put together with wider environmental concerns (eg: ocean-oxygen levels could be accidentally depleted, or toxic algae may start to grow) seems clear to me that this is not a form of geoengineering Eazrah should be considering putting into action.
I'll shortly be leaving Planet Crooter, and my quest for promising geoengineering continues.
Hi, in my opinion iron fertilisation is one of the worst CDR geoengineering processes to be considered, as it has so many environmental impacts and can lead to the extinction of many marine species.
ReplyDeletethanks for your comment, Maria.
DeleteI agree - it seems risky and unpredictable. And to see any significant result from it, a large portion of the oceans would need to be fertilized continuously, increasing these risks. All too dangerous if you ask me.
Did those Crooterians say anything about the effect of DMS emissions resulting from iron fertilisation?
ReplyDelete